The Boris-Lawrie Lab
Research Summary
Considering the fundamental discoveries made in studies of retroviruses, an obvious pattern emerges: Retroviruses open windows to see how cells work. Beyond discovery, studies at the virus-host interface expose new strategies for diminishing the severity of infections and proliferation of transformed cells.
The genetic basis of cancer, the fundamental principles of DNA transcription and mRNA biogenesis, genetic engineering of vectors for gene transfer; and host-pathogen adaptation in human and animal species, especially since HIV-AIDS, are a few of the lessons learned from molecular retrovirology.
Because retroviruses are dependent on cellular machineries to replicate their small RNA genome, and since their DNA copy assimilates into the chromosomes of any infected cell and all of their biological needs are provided by host cellular machinery, retroviruses provide a fascinating microcosm of cell biology.
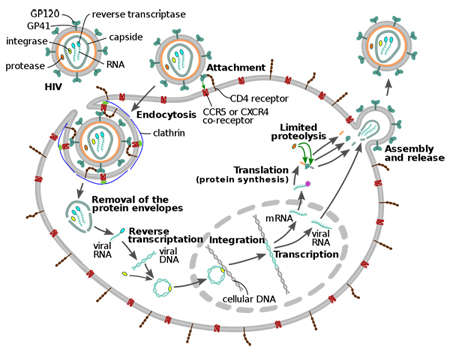
Our laboratory is dedicated to discovering the RNA regulatory mechanisms necessary for retroviruses to co-opt cellular gene expression machineries. The cell’s posttranscriptional machineries, plus retroviral and cellular nucleic acid substrates, are the raw materials for our investigations. Genetic tools and genomic, biochemical and biophysical approaches define commonalities between RNA features and have defined specific RNA binding proteins both cells and viruses use to adjust the functional activity of one RNA to meet multiple purposes. We now know dynamic changes in viruses' RNPs enable a single RNA sequence to encode mRNA template for translation or storage and viral genome packaged and released in viral particles or cellular vesicles.
By comparative analysis, we have unveiled stealth ribonucleoprotein complexes that viruses adapt to support their own proliferation. Functionally, these RNPs are usurped from a cell-protective role to a virus-promoting role. We are successfully defining which cognate features in cellular RNAs are recapitulated in viral RNAs, and which regulatory interactions are responsible for physiological control of gene expression. Necessarily, the broadened impact of our research stretches to how defects in mRNA regulatory pathways contribute to cancer and infectious disease.